Kariko and colleagues discovered that incorporation of nucleoside base modifications such as N1-Methylpseudouridine significantly increase antigen expression and prevent excessive innate immune responses towards synthetic mRNA. This discovery led the foundation for successful mRNA-based vaccine development[1-5].
The concept has been successfully applied to saRNA (Fig. 1): 5-Methylcytidine-modified saRNA shows increased transfection & translation efficiency as well as reduced innate immune responses compared to its unmodified counterpart[6-9]. The modification is conveniently introduced by 5-Methyl-CTP via T7 RNA polymerase-mediated in vitro transcription (Fig. 1B, Tab. 1).
An increased saRNA effectiveness is an important step towards the development saRNA vaccines that promise lower- and single- dose vaccines thus reducing both vaccination side effects and costs[10-13].
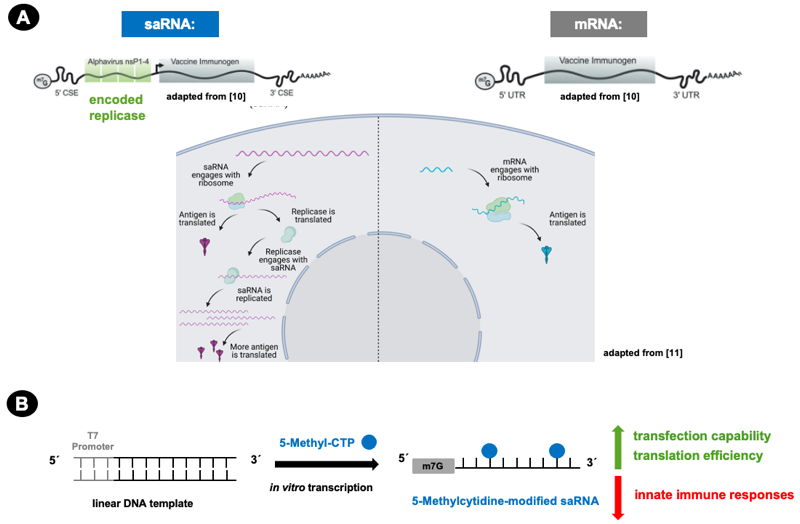
Figure 1: Advantages of self-amplifying RNA (saRNA) are fully exploited by 5-Methylcytidine modification.
A) Encoded Alphavirus replicase leads to intracellular saRNA amplification and thus increased antigen expression compared to mRNA (adapted from Bloom et al.[10] and Blakney et al.[11]).
m7G: 7-methylguanosine cap, nsP 1-4: nonstructural protein 1-4, CSE: conserved sequence element, UTR: untranslated region
B) In vitro transcribed 5-Methylcytidine-modified saRNA shows increased transfection and translation efficiency as well as reduced innate immune responses[5-8].
m7G: 7-methylguanosine cap
| Compound | Cat. No. | Amount | Purity | Accompanying documentation |
|---|---|---|---|---|
| Small scale R&D amounts | ||||
| 5-Methyl-CTP | NU-1138S | 10 µl (100 mM) | >/= 95 % (HPLC) | CofA |
| NU-1138L | 5x 10 µl (100 mM) | |||
| Bulk amounts for production | ||||
| 5-Methyl-CTP | NU-1138-THR-1 | 1 ml (100 mM) | >/= 99 % (HPLC) | CofA CoO Country of Origin |
| NU-1138-THR-10 | 10 ml (100 mM) | |||
| NU-1138-THR-100 | 100 ml (100 mM) | |||
| NU-1138-THR-CSTM | Custom specific bulk amount | |||
[1] Karikó et al. (2005) Suppression of RNA Recognition by Toll-like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA. Immunity 23:165.
[2] Karikó et al. (2008) Incorporation of Pseudouridine into mRNA Yields Superior Nonimmunogenic Vector With Increased Translational Capacity and Biological Stability. Mol. Ther. 16(11):1833.
[3] Anderson et al. (2011) Nucleoside modifications in RNA limit activation of 2’-5’-oligoadenylate synthetase and increase resistance to cleavage by RNAse L. Nucleic Acids Research 39(21):9329.
[4] Andies et al. (2015) N1-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J. Control. Release 217:337.
[5] Morais et al. (2021) The Critical Contribution of Pseudouridie to mRNA COVID-19 Vaccines. Frontiers in Cell and Developmental Biology 9:1.
[6] McGee et al. (2024) Complete substitution with modified nucleotides in self-amplifying RNA suppresses the interferon response and increases potency. Nature Biotechnology: https://doi.org/10.1038/s41587-024-02306-z.
[7] Azizi et al. (2024) Self-amplifying RNAs generated with the modified nucleotides 5-methylcytidine and 5-methyluridine mediate strong expression and immunogenicity in vivo. NAR Molecular Medicine 1(2) :1.
[8] Aboshi et al. (2024) Safety and immunogenicity of VLPCOV-02, a SARS-CoV-2 self-amplifying RNA vaccine with a modified base, 5-methylcytosine. iScience 27 :108964.
[9] Komori et al. (2023) Incorporation of 5-Methylcytidine alleviates innate immune response to self-amplifying RNA vaccine. bioRxiv preprint doi: https://doi.org/10.1101/2023.11.01.565056.
[10] Bloom et al. (2021) Self-amplifying RNA vaccines for infectious diseases. Gene Therapy 28 :117.
[11] Blakney et al. (2021) An Update on Self-Amplifying mRNA Vaccine Development. Vaccines (Basel) 9(2) :97.
[12] Comes et al. (2023) Rise of RNA machines – self-amplification in mRNA vaccine design. Trends in Biotechnology 41(11) :1417.
[13] Schmidt et al. (2023) Self-Amplifying RNA Vaccine Candidates: Alternative Platforms for mRNA Vaccine Development. Pathogens 12:138.